HSV gene therapy company Telaria to focus on skin diseases
Repeat has announced the launch of Telaria, a herpes simplex virus (HSV) gene therapy company targeting rare skin diseases.
It is the second of the Replay manufacturing companies to use the high-capacity HSV delivery vector, synHSV. The co-founders of Telaria are scientists, entrepreneurs and experts in the field of genetic skin diseases.
In addition to Joe Glorioso, Telaria is co-founded by John McGrath, an expert in genetic skin diseases, and Alexander Silver, co-founder and chairman emeritus of the Epidermolysis Bullosa Research Partnership. Jakub Tolar, McKnight Professor Emeritus in the Departments of Pediatrics, Blood and Marrow Transplantation, and Cell Therapy at the University of Minnesota Medical School, is a senior advisor to the company.
Telaria is the second of Replay’s four synHSV gene therapy companies, and its launch follows Eudora, a genetic retinal disease company. Replay’s distinctive corporate structure separates technology development from product development in the disease treatment companies.
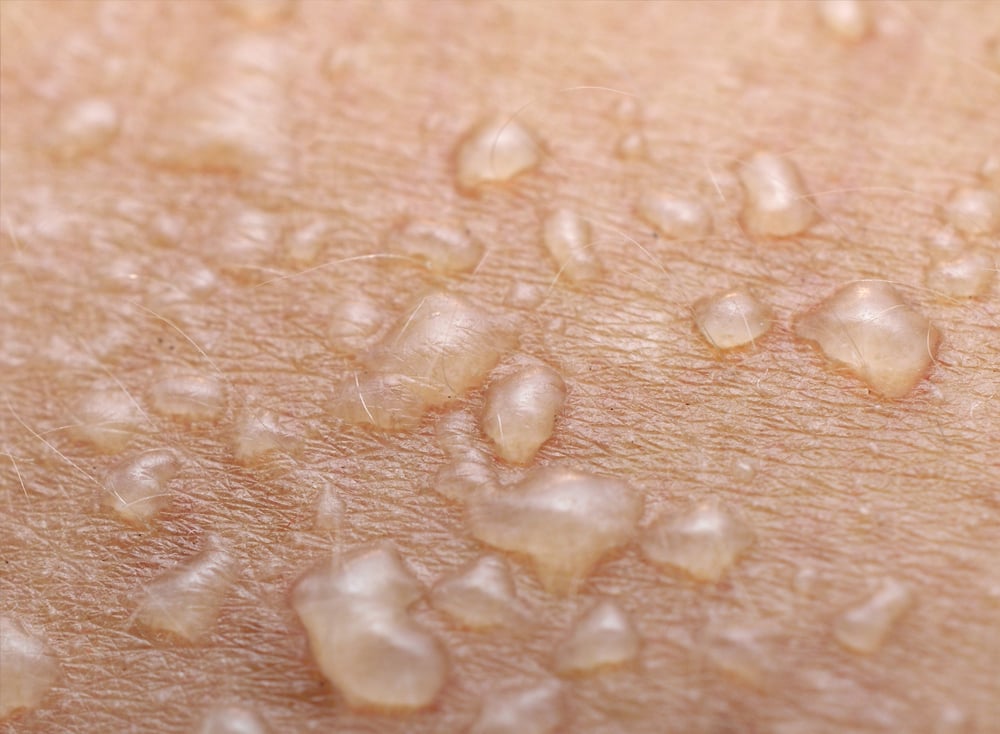
The main indication in Telaria’s pipeline is recessive dystrophic epidermolysis bullosa (RDEB), a genetic skin condition that makes the skin fragile and blisters easily. RDEB involves extensive blistering that can lead to scarring, severe pain, and disfigurement. It can affect multiple internal organs and cause serious medical problems, which can include chronic inflammation and squamous cell carcinoma.
Benefits for patients
There is currently no cure for the approximately 50,000 RDEB patients worldwide, and palliative wound care is the current standard of care. Gene therapy that modifies the disease and is able to heal wounds faster or prevent their formation will be a significant advantage for patients.
Replay’s synHSV technology is a high-payload gene-deleted HSV-1 vector capable of carrying up to eight times the payload of adeno-associated virus (AAV) vectors. This allows the delivery of genes too large to fit into AAV and facilitates polygenic gene therapy. Replay is also developing an HSV vector capable of delivering up to 30 times the AAV payload.
Adrian Wolfson, executive chairman, president and co-founder of Replay, said: “The skin is an attractive target for gene therapy and is the largest and most accessible organ. Telaria, which is exclusively focused on developing transformative treatments for rare genetic skin diseases and is the second of our four synHSV product companies, represents another significant step towards building a solid company with the potential to shape the future of genomic medicine by addressing some of the most significant problems that currently limit the progress of clinical medicine.”
There is no approved medicine
McGrath said Telaria is developing a potentially best-in-class polygenic gene therapy to more quickly and effectively treat existing skin lesions and prevent new ones from forming.
Silver said, “There are currently no approved medications for people with RDEB, and I know from my personal experience that the current standard of care is limited and does not provide long-term, sustainable benefit to patients.
“Replay’s synHSV technology, which enables the delivery of large DNA to the skin using a differentiated payload capacity, has the potential to disrupt the field of gene therapy for genetic skin diseases and provide patients with the treatments they need as quickly and safely as possible.”
https://www.labiotech.eu/trends-news/hsv-gene-therapy-company-telaria-skin-diseases/ HSV gene therapy company Telaria to focus on skin diseases